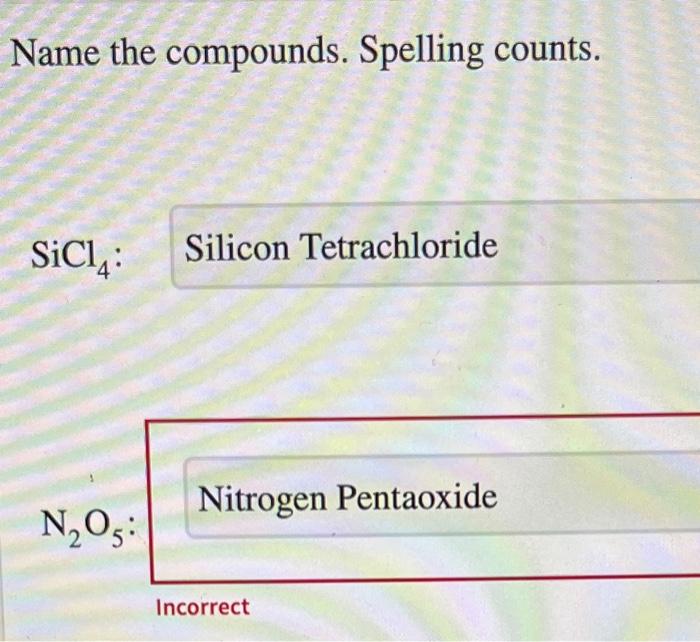
Name the compounds. spelling counts. – Beginning with the fundamental concept of naming compounds, this comprehensive guide delves into the intricacies of organic chemistry, unraveling the complexities of nomenclature, isomerism, functional groups, chemical reactions, and spectroscopy. Embark on a journey of discovery as we explore the fascinating world of organic compounds, where precision in spelling holds paramount importance.
Nomenclature, the systematic naming of compounds, forms the cornerstone of organic chemistry, enabling scientists to communicate precisely about the structures and properties of these molecules. Understanding the rules and conventions established by the International Union of Pure and Applied Chemistry (IUPAC) is essential for effective communication and accurate identification of compounds.
Nomenclature

The International Union of Pure and Applied Chemistry (IUPAC) has established guidelines for naming compounds to ensure consistency and clarity in scientific communication. These guidelines provide a systematic approach to naming organic and inorganic compounds based on their structure and functional groups.
The IUPAC nomenclature system follows a set of rules that consider the number of carbon atoms, the presence of functional groups, and the type of bonding between atoms. The systematic name of a compound typically consists of three parts: a prefix, a parent name, and a suffix.
The prefix indicates the number of carbon atoms in the parent chain, the parent name identifies the type of compound based on its functional group, and the suffix indicates the presence of specific substituents or groups attached to the parent chain.
Alkanes, Name the compounds. spelling counts.
Alkanes are saturated hydrocarbons, meaning they contain only single bonds between carbon atoms. The prefix for alkanes is based on the number of carbon atoms in the parent chain. The parent name for alkanes ends in “-ane.” For example, the systematic name for a hydrocarbon with six carbon atoms is hexane.
Alkenes
Alkenes are unsaturated hydrocarbons, meaning they contain at least one double bond between carbon atoms. The prefix for alkenes is based on the number of carbon atoms in the parent chain. The parent name for alkenes ends in “-ene.” For example, the systematic name for a hydrocarbon with four carbon atoms and one double bond is butene.
Alkynes
Alkynes are unsaturated hydrocarbons, meaning they contain at least one triple bond between carbon atoms. The prefix for alkynes is based on the number of carbon atoms in the parent chain. The parent name for alkynes ends in “-yne.” For example, the systematic name for a hydrocarbon with five carbon atoms and one triple bond is pentyne.
Alcohols
Alcohols are organic compounds that contain a hydroxyl group (-OH) attached to a carbon atom. The prefix for alcohols is based on the number of carbon atoms in the parent chain. The parent name for alcohols ends in “-ol.” For example, the systematic name for an alcohol with three carbon atoms is propanol.
Aldehydes
Aldehydes are organic compounds that contain a carbonyl group (C=O) at the end of a carbon chain. The prefix for aldehydes is based on the number of carbon atoms in the parent chain. The parent name for aldehydes ends in “-al.”
For example, the systematic name for an aldehyde with four carbon atoms is butanal.
Ketones
Ketones are organic compounds that contain a carbonyl group (C=O) within a carbon chain. The prefix for ketones is based on the number of carbon atoms in the parent chain. The parent name for ketones ends in “-one.” For example, the systematic name for a ketone with three carbon atoms is propanone.
Carboxylic Acids
Carboxylic acids are organic compounds that contain a carboxyl group (-COOH) at the end of a carbon chain. The prefix for carboxylic acids is based on the number of carbon atoms in the parent chain. The parent name for carboxylic acids ends in “-oic acid.”
For example, the systematic name for a carboxylic acid with three carbon atoms is propanoic acid.
Esters
Esters are organic compounds that contain an ester group (-COOR) formed by the reaction of a carboxylic acid with an alcohol. The prefix for esters is based on the number of carbon atoms in the parent chain. The parent name for esters ends in “-oate.”
For example, the systematic name for an ester formed from the reaction of propanoic acid and methanol is methyl propanoate.
Isomerism: Name The Compounds. Spelling Counts.

Isomerism is a phenomenon in which compounds with the same molecular formula have different structural or spatial arrangements of atoms. These compounds are known as isomers and exhibit different physical and chemical properties.
Types of Isomers
Structural Isomers
Structural isomers have the same molecular formula but differ in the arrangement of their atoms. There are three main types of structural isomers:
- Chain isomers:Differ in the order of carbon atoms in the main chain.
- Position isomers:Differ in the position of a functional group or substituent on the carbon chain.
- Functional group isomers:Have different functional groups despite having the same molecular formula.
Stereoisomers
Stereoisomers have the same molecular formula and connectivity but differ in the spatial arrangement of their atoms in three-dimensional space. There are two main types of stereoisomers:
- Geometric isomers:Have different spatial arrangements of atoms or groups around a double bond or ring.
- Optical isomers:Non-superimposable mirror images of each other.
Functional Groups
Functional groups are specific groups of atoms within a molecule that are responsible for its characteristic chemical properties. They determine the reactivity, solubility, and other physical and chemical properties of organic compounds.
The common functional groups in organic chemistry include:
- Alkanes: Contain only carbon and hydrogen atoms, with single bonds between the carbon atoms.
- Alkenes: Contain carbon-carbon double bonds.
- Alkynes: Contain carbon-carbon triple bonds.
- Alcohols: Contain a hydroxyl group (-OH) bonded to a carbon atom.
- Ethers: Contain an oxygen atom bonded to two carbon atoms.
- Aldehydes: Contain a carbonyl group (C=O) bonded to a hydrogen atom.
- Ketones: Contain a carbonyl group (C=O) bonded to two carbon atoms.
- Carboxylic acids: Contain a carboxyl group (-COOH) bonded to a carbon atom.
- Esters: Contain a carbonyl group (C=O) bonded to an oxygen atom, which is in turn bonded to a carbon atom.
- Amides: Contain a carbonyl group (C=O) bonded to a nitrogen atom.
Each functional group has its own characteristic reactivity and properties. For example, alcohols can undergo dehydration reactions to form alkenes, while carboxylic acids can react with bases to form salts.
Chemical Reactions
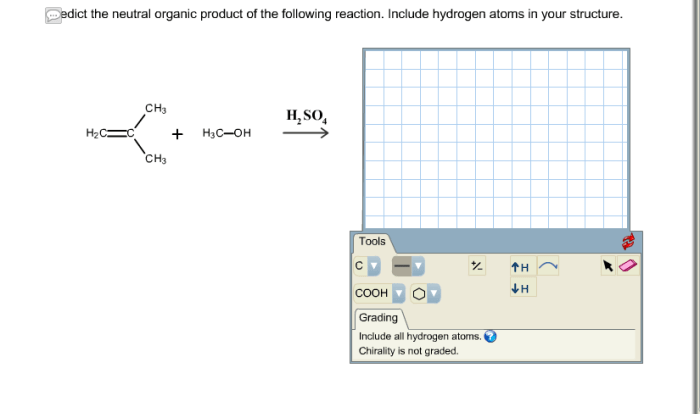
Chemical reactions are processes that involve the rearrangement of atoms and the formation of new substances. Organic compounds can undergo a variety of chemical reactions, including addition, substitution, elimination, and redox reactions.
- Addition reactionsinvolve the addition of an atom or a group of atoms to a molecule. For example, the addition of hydrogen to an alkene results in the formation of an alkane.
- Substitution reactionsinvolve the replacement of an atom or a group of atoms in a molecule with another atom or group of atoms.
For example, the substitution of a hydrogen atom in an alkane with a chlorine atom results in the formation of an alkyl halide.
- Elimination reactionsinvolve the removal of an atom or a group of atoms from a molecule. For example, the elimination of a hydrogen atom and a hydroxide ion from an alcohol results in the formation of an alkene.
- Redox reactionsinvolve the transfer of electrons between atoms or molecules. For example, the oxidation of an alcohol to a ketone results in the transfer of two electrons from the alcohol to the oxidizing agent.
Each type of chemical reaction has its own unique mechanism. The mechanism of a reaction is the step-by-step process by which the reaction occurs. The mechanism of a reaction can be determined by using a variety of techniques, including spectroscopy, mass spectrometry, and nuclear magnetic resonance (NMR) spectroscopy.
Spectroscopy

Spectroscopy is the study of the interaction of electromagnetic radiation with matter. It is a powerful tool for identifying and characterizing organic compounds because it can provide information about the structure, composition, and functional groups present in the molecule.
Infrared (IR) Spectroscopy
IR spectroscopy measures the absorption of infrared radiation by a molecule. The absorption bands in an IR spectrum correspond to the vibrational frequencies of the bonds in the molecule. This information can be used to identify the functional groups present in the molecule and to determine the structure of the molecule.
Nuclear Magnetic Resonance (NMR) Spectroscopy
NMR spectroscopy measures the absorption of radio waves by the nuclei of atoms in a molecule. The absorption bands in an NMR spectrum correspond to the different types of atoms in the molecule and their chemical environment. This information can be used to determine the structure of the molecule and to identify the functional groups present in the molecule.
Mass Spectrometry (MS)
MS measures the mass-to-charge ratio of ions produced from a molecule. The mass-to-charge ratio of an ion is characteristic of the molecule from which it was produced. This information can be used to identify the molecular weight of the molecule and to determine its structure.
Question & Answer Hub
Why is spelling so important in naming compounds?
Spelling errors can lead to confusion and misinterpretation, potentially resulting in incorrect identification or synthesis of compounds. Accurate spelling ensures clear communication and understanding among chemists.
What are the key rules for naming alkanes?
Alkanes are named based on the number of carbon atoms in the parent chain, using the suffix “-ane.” The prefixes “meth-,” “eth-,” “prop-,” “but-,” and so on, indicate the number of carbon atoms.
How do you differentiate between structural and stereoisomers?
Structural isomers have the same molecular formula but different connectivity of atoms, while stereoisomers have the same molecular formula and connectivity but differ in the spatial arrangement of atoms.