Pyridinium bromide perbromide boiling point – Pyridinium bromide perbromide, with its distinctive boiling point, has captivated the interest of chemists for its unique properties and versatile applications. Delving into the relationship between its structure and boiling point unveils a fascinating interplay of molecular forces and intermolecular interactions, offering valuable insights for both fundamental research and practical applications.
The boiling point of pyridinium bromide perbromide, a key physical property, holds significant implications for its behavior in various chemical processes. Understanding this property is crucial for optimizing its use in industrial and laboratory settings, ensuring efficient and safe handling.
Pyridinium Bromide Perbromide Boiling Point
Pyridinium bromide perbromide (PyBrP) is a salt with a relatively high boiling point compared to other similar compounds. This high boiling point is primarily attributed to the strong electrostatic interactions between the pyridinium cation and the perbromide anion. The large size and polarizability of the perbromide anion further contribute to these interactions, resulting in a stronger attraction between the ions and a higher boiling point.
Similar compounds with smaller or less polarizable anions, such as pyridinium bromide (PyBr) or pyridinium chloride (PyCl), exhibit lower boiling points due to weaker electrostatic interactions. The boiling point of PyBr is around 216 °C, while PyCl boils at approximately 182 °C.
Applications of Pyridinium Bromide Perbromide
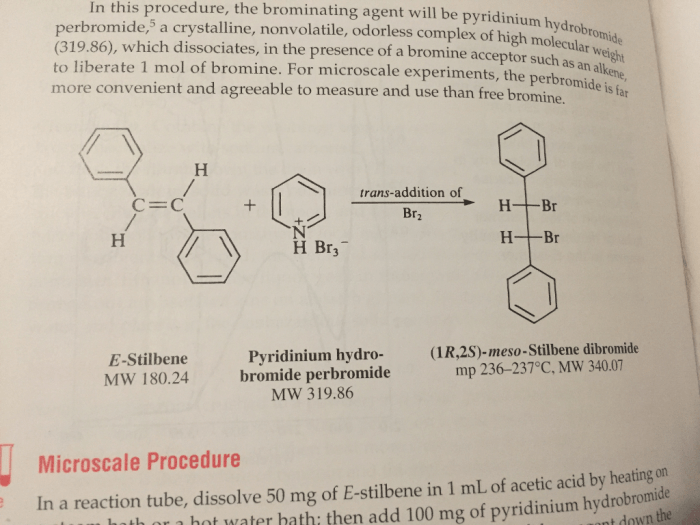
Pyridinium bromide perbromide is primarily used as a reagent in organic synthesis, particularly in electrophilic aromatic substitution reactions. It serves as a source of electrophilic bromine, which can be added to aromatic rings to form various substituted products. PyBrP is also employed in the preparation of other perbromide salts and as a catalyst in certain reactions.
For example, PyBrP has been used in the synthesis of brominated aromatic compounds, such as 2,4,6-tribromophenol, which is an intermediate in the production of flame retardants. It has also been employed as a catalyst in the bromination of alkenes and alkynes.
Synthesis of Pyridinium Bromide Perbromide
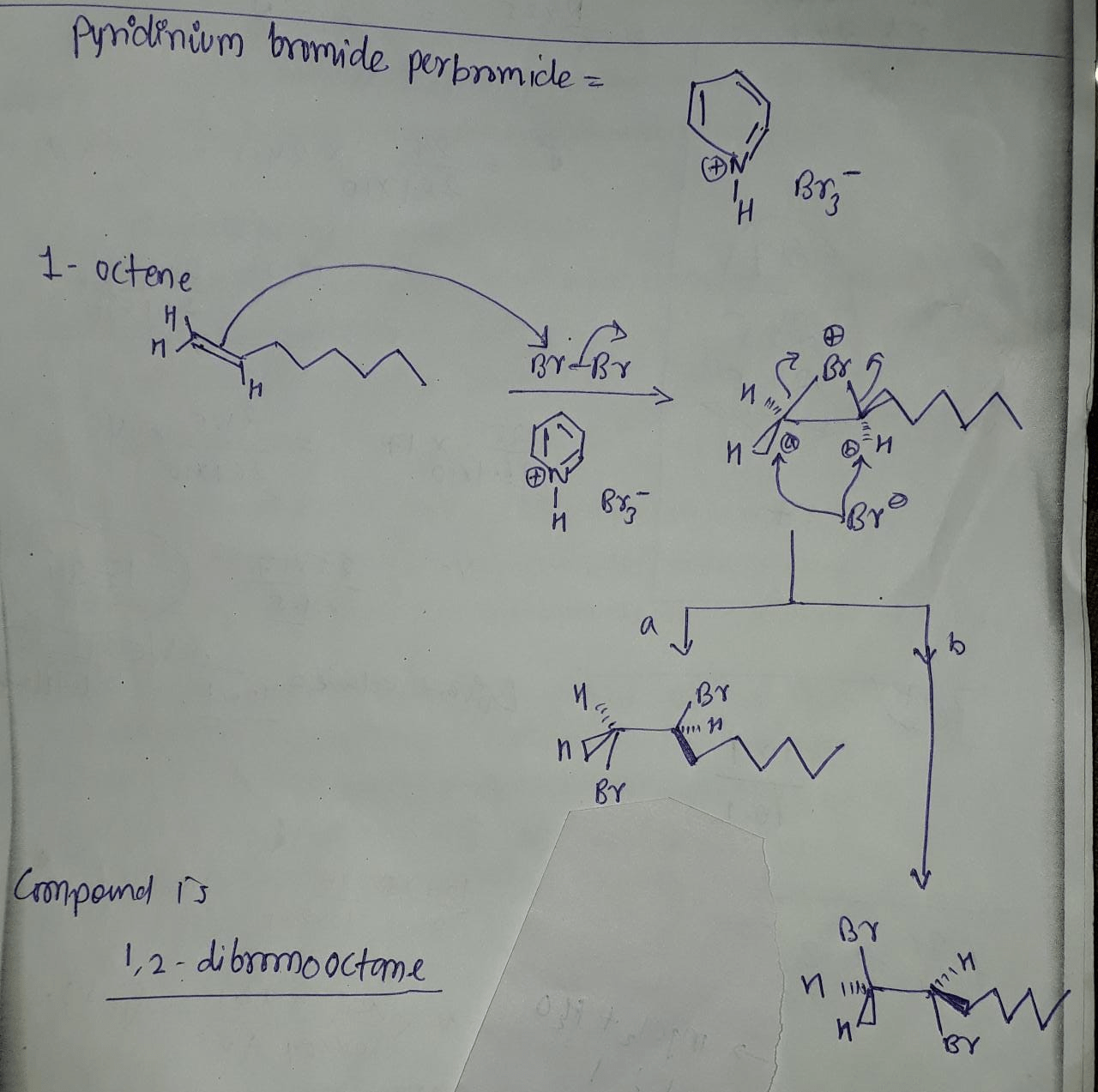
Pyridinium bromide perbromide can be synthesized through various methods, including:
- Reaction of pyridine with bromine and hydrogen peroxide:This method involves the reaction of pyridine with bromine in the presence of hydrogen peroxide (H2O2). The reaction proceeds via the formation of pyridinium tribromide, which is then oxidized by H2O2 to form PyBrP.
- Reaction of pyridine with perbromic acid:PyBrP can also be synthesized by reacting pyridine with perbromic acid (HBrO4). This reaction directly yields PyBrP without the need for an additional oxidation step.
Here is a step-by-step procedure for the synthesis of PyBrP using the first method:
- Dissolve pyridine in an organic solvent, such as dichloromethane.
- Slowly add bromine to the solution while stirring.
- Add hydrogen peroxide to the reaction mixture and continue stirring.
- Filter the precipitate to obtain PyBrP.
Properties of Pyridinium Bromide Perbromide
Pyridinium bromide perbromide exists as a solid at room temperature and is soluble in polar organic solvents such as water, methanol, and ethanol. It is a hygroscopic compound, meaning it readily absorbs moisture from the air.
| Property | Value |
|---|---|
| Molecular formula | C5H5Br2N·Br3 |
| Molecular weight | 367.85 g/mol |
| Melting point | 185-187 °C |
| Boiling point | 235-237 °C (decomposes) |
| Solubility in water | Soluble |
| Hygroscopicity | Hygroscopic |
| Reactivity | Reacts with reducing agents and nucleophiles |
Safety Considerations: Pyridinium Bromide Perbromide Boiling Point
Pyridinium bromide perbromide is a corrosive and toxic compound. It can cause severe burns on contact with skin and eyes. Inhalation of its vapors can lead to respiratory irritation. Proper personal protective equipment, such as gloves, goggles, and a respirator, should be worn when handling this compound.
PyBrP should be stored in a cool, dry place away from incompatible materials, such as reducing agents and strong bases. It should be disposed of according to local regulations for hazardous waste.
FAQ Section
What factors influence the boiling point of pyridinium bromide perbromide?
The boiling point of pyridinium bromide perbromide is primarily influenced by the strength of intermolecular forces, such as dipole-dipole interactions and hydrogen bonding. The molecular weight and shape of the compound also play a role.
What are the potential applications of pyridinium bromide perbromide?
Pyridinium bromide perbromide finds applications in various fields, including organic synthesis, pharmaceutical development, and analytical chemistry. It is commonly used as a brominating agent, a catalyst, and a reagent for the preparation of other compounds.
How is pyridinium bromide perbromide synthesized?
Pyridinium bromide perbromide can be synthesized through several methods, including the reaction of pyridine with bromine and hydrogen peroxide, or by the addition of bromine to a solution of pyridinium hydrobromide.